Products
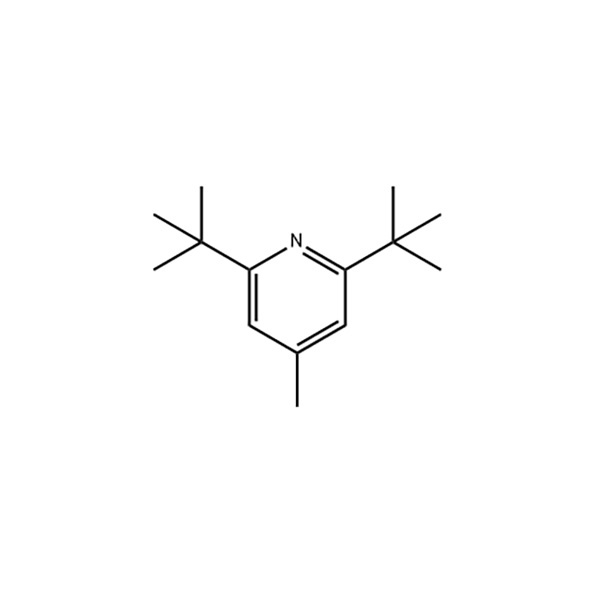
2,6-Di-tert-butyl-4-methylpyridine
Formulae structurales
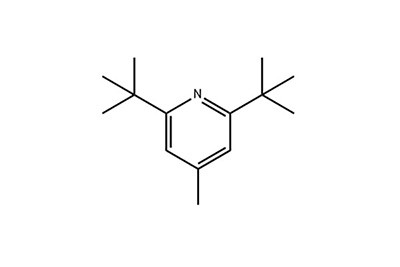
Aspectus: crystallus albus acicularis
Densitas: 1,476 g/cm3
Punctum liquescens: 31-32
Fervens punctum: 148-153℃ (12.6kPa)
Index refractivus: N20 /D 1.4763(lit.)
Aequaliter Nubila. 183 °F
Repono conditiones: 2-8°C
Acidum coefficiens (pKa): 6.88±0.10(Praedictum)
Salus Data
Pertinet ad generale navis
Consuetudines code: 2942000000
Detrahetur tributum exportare (%): 13%
Applicationem
Synthetica media organica, res sterically impedita, basis non-nucleophilica, quae inter Brnstedum (protonica) et Ludovicum acida distinguit.Directum altum dat conversionem aldehydes1 et ketones1,2 ad triflationes vinyllorum.
2,6-Di-tert-butyl-4-methylpyridine compositio organica cum formulae hypothetica C14H23N, media maximus in synthesi organica, maxime adhibita in mediis pharmaceuticis, synthesi organica, menstrua organica, etiam applicari potest in productione, pesticide. confectio et odores, etc.
Productio methodo
1. Fac 2,6 di-tert-butyl-4-methylbenzyl trifluoromethanesulfonate In a 100-mL lagena triplici instructa cum introductorio NITROGENIO, pressione infundibuli constanti, vecte electromagnetico agitante et glaciem condensatorem cum tubo sicco, adde 24.2 g (0.2 mol) trimethylethyi phthalidis chloridi et 3.7 g (0.05 mol) tert-butanol.Reactio mixtura in balneis olei ad 85°C calefacta est.Inde 15 g (0.1 mol) acidum trifluoromethanesulfonicum plus quam 15 minuta addita est.Reactio per X minuta continuata est, et leve reactionis fuscae productum in balneum glaciei refrigeratum est et in 100 ml frigidum aetherem funditur.Fucum praecipitatum percolantur et exsiccentur ad 9.6 g (54%) de stridore morato sale.(Nulla purificatio requiritur: ad gradum praeparationis proximum bis ab chloroformi ad tetrachloride carbonii 3:1) recrystallizare ad crystallos acustiles hyalinosas.
2. Praeparatio 2,6-Di-tert-butyl-4-methyl-vizini sub agitatione, suspensionis 10 g (0.028 mol) salis crudi vizini in 200 mL 95% ethanoli frigoris ad 60°C erat addita semel ad 100 mL ammoniaci congesti frigidum ad 60°C in lagona 1-sexterna.Reactio continuata est 60°C per 30 minuta et 40°C pro 2 horis, quo tempore suspensio dissoluta est.Tum, reactionem mixturam in 500 ml 2% hydroxidis sodium solutionis sodium infusam lente ad cella temperiei calefacta est.Extrahi cum .4-100 ml pentanis, excerptis coniunctis, 25 ml solutionis sodium chloridi saturati loti, 100 ml gyratorii evaporatoris concentrati pentanei.